BYU chemist Dan Simmons has unlocked a secret of painkillers, providing better relief to millions and bringing renown to BYU.
Before settling down for the night in the late 1990s, Trudy Woods Simmons, ’79, would look into her medicine cabinet and consider the choice she faced as an arthritis sufferer. Traditional aspirin-like drugs such as ibuprofen would take away some of the pain and inflammation in her knees and hips, but after long periods of regular use, they also caused nagging stomach pains. It was worse for others—between 8,000 and 15,000 people died annually from bleeding ulcers resulting from long-term painkiller use.
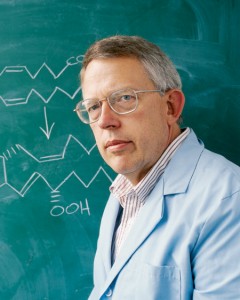
When Dan Simmons discovered the COX-2 enzyme, which causes pain and inflammation, he knew he had found something extraordinary. Since that day, doctors have prescribed drugs developed from Simmons’ discovery to millions of patients. Photo by Bradley H. Slade, ’94.
But when Simmons swallowed her nightly pill and went to bed, she was comforted by the knowledge that a new kind of pain reliever was on its way. Of course, she knew a little bit more than most people about this coming revolution in pain relief. The man snoring beside her ignited it in his BYU laboratory about a decade earlier.
Today, just about everyone in America knows someone who regularly takes one of the painkillers that resulted from Daniel Simmons’ discovery, one of the most significant ever made at BYU. The first to market was Celebrex in 1999, produced by a company that has since been acquired by Pfizer. The drug, promoted by ubiquitous television commercials urging America’s 20 million arthritis sufferers to “celebrate,” was prescribed by physicians 17 million times in its first year—about 55,000 a day—making it the fastest-selling drug in history to that point. Merck’s Vioxx arrived a few months later, and doctors wrote 52 million prescriptions for it in its first 2.5 years on the American market.
“Daniel Simmons is among the foremost research scientists in the world today,” says Regina M. Botting, a research fellow of the William Harvey Research Institute in London.
“He brings honor to Brigham Young University and, by his presence, enhances its reputation in both research and scholastic fields.”
Recognition, Stature, and a “Hideous” Choice
Daniel L. Simmons, ’78, a BYU professor of chemistry and biochemistry, grew up near Rock Canyon on what were then the outskirts of Provo. He was one of those children who asked for chemistry sets for Christmas and tried to examine snowflakes under a microscope. After earning bachelor’s and master’s degrees in zoology at BYU, he pursued a doctorate in oncology at the University of Wisconsin—Madison. He sought a demanding environment, and he found it.

Photo by Bradley H. Slade, ’94
“At Wisconsin I had it pounded into me that you bang it out no matter what is in the way,” he says. “You do the experiment, even if you have to walk across campus and borrow some material—you get it done today.” The urgency was a necessity in the naturally competitive scientific arena, where honors go only to those who finish first.
Simmons was competitive enough to earn a post-doctoral fellowship in cancer research at Harvard in 1986, an experience that would lead to many productive research projects and one life-altering decision.
About a year before his Harvard fellowship was scheduled to end, Simmons prepared for the next step in his career by sending out feelers for faculty positions at universities. Coincidentally, a position had just opened in BYU’s Department of Chemistry and Biochemistry, and Simmons was immediately invited to an interview. Ahead of his own schedule, he accepted the offer that followed.
Aware that the BYU position would not be highly regarded by his laboratory colleagues, Simmons was a little nervous about sharing the surprise announcement. He planned to tell them at one of the group’s semi-weekly “teas.” In a tradition brought to Harvard from Oxford by James D. Watson, who helped identify the structure of DNA in 1953, the researchers gathered Tuesday and Thursday afternoons for brie, hummus, and political chatter. Glancing at his four fellow post-docs, who would go on to accept positions at Yale, Princeton, and Harvard Medical School, Simmons took a deep breath and announced that he was going to BYU.
“Immediate silence,” Simmons recalls of their reaction. “The communal embarrassment of this ‘hideous’ position was palpable. They thought I was the most idiotic person there.”

Photo by Bradley H. Slade, ’94
Simmons’ best friend in the lab finally offered a tight-lipped “congratulations,” and the conversation quickly moved to more comfortable topics. The friend also moved quickly, lining up an interview for Simmons at Harvard Medical School, where he had already accepted a position. Simmons was invited to accept a full-time slot and the opportunity to start in a few months.
Faced with a new alternative, Simmons admits he had second thoughts about accepting the BYU job. Not only did the university lack any semblance of the scientific reputation of Harvard, the research infrastructure at BYU then paled in comparison. Simmons was facing two options: a bare-bones laboratory with no graduate students, little money, and a heavy undergraduate teaching load, or a fully outfitted laboratory with eager graduate students and post-doctoral fellows, startup funding, and little teaching responsibility.
“I went through a lot of introspection,” he says. For reasons he has chosen to keep personal, he elected to return to BYU.
“Dan was one of the first DNA researchers to come to BYU and be that well recognized, to come from that kind of a background, with that stature,” says Earl M. Woolley, ’66, then Simmons’ department chair. “DNA-based research hadn’t yet hit the mass market.”
Nabbing the Culprit of Pain
Trying to nail down the cellular chemicals and components responsible for illnesses can be like attempting to identify a ring of pickpockets in a packed shopping mall. Thousands of “innocent subjects” swirl around a few “guilty parties,” masking the crimes with their routine activities. Like a sign flashed by a ringleader, one small signal in a cell jump-starts a cascade of events that ultimately lead to problems.
But mutated genes or malfunctioning proteins can be much more dangerous than a pickpocket, even leading to death. Fortunately, unlike detectives who arrest one criminal at a time, medical sleuths can identify and halt certain disorders once and for all, as they have done with polio and smallpox.
Arriving at BYU in the summer of 1989, Simmons brought with him genetic samples that were part of a Harvard project he conducted in the laboratory of decorated scientist Raymond Erikson. At Harvard Simmons had identified some samples that are activated when cells become cancerous. He isolated others, but it was unclear what, if any, role they have in cancer. He borrowed a graduate student, Weilin Xie, ’91, from a fellow faculty member and embarked on his independent research career. The duo painstakingly sequenced the DNA contained in the unknown genetic segments and compared the data with DNA from genes whose functions were known.
There’s a reason crowds don’t gather to watch science unfold—it’s a lot of sitting still, thinking hard, and trying again. But Simmons toiled with the quiet urgency of a detective methodically working through a crowd, comparing the individuals he encounters to the descriptions of the suspects.
Photo by Bradley H. Slade, ’94
Intrigued by the persistent appearance of its activity in cancer, Simmons focused on a gene segment he unimaginatively dubbed CEF-147. Assisted by Xie, he began the meticulous process of manually sequencing its DNA to clearly identify the gene and its role. They carefully elucidated each of the thousands of As, Ts, Gs, and Cs that make up the code.
Simmons remembers that few people were around his Widtsoe Building lab on the day he completed the sequencing. “It was like a tomb,” he says.
He typed the finished sequence into the GenBank database, where known genes’ descriptions are cataloged (now the repository of the Human Genome Project). This step was similar to running the fingerprint of an anonymous captured crook through a database of convicts, searching for a match.
Simmons was intrigued when he saw that CEF-147 contained the code for a protein that interacts with aspirin. Scientists didn’t know how the centuries-old painkiller worked until 1971, when British researcher John R. Vane discovered that aspirin succeeds by preventing an enzyme—a protein that initiates biochemical reactions—from creating molecules that cause pain and inflammation. The enzyme is called cyclooxygenase, or COX, for short, and in 1982 Vane was awarded the Nobel Prize for the discovery.
Although Simmons’ enzyme bore a similarity to COX, the computer revealed that CEF-147 had significant differences from the known form of any previously discovered enzyme. The clues were mounting, and Simmons began to realize that he had nailed a new culprit, one that was involved in cancer as well as pain and inflammation. And he knew that with this information, other researchers could develop a drug to knock the enzyme out of commission, thus relieving pain with fewer side effects.
“When I saw the first data that it was a new cyclooxygenase, I nearly rocketed through the roof,” Simmons says. “It was just one of those instantaneous things that you have rarely in science.”
Xie remembers the exultant screams that broke the Widtsoe Building silence that day. “I had never seen Dr. Simmons act like that,” he says. “Since biological research is so complex, there are few times that you are so sure on something. There were no doubts in our minds that the finding was very significant. We knew that new drugs could and should be made from the discovery.”
One of the first people Simmons told about his findings was his department chair. “He wanted to close the door, and he was almost whispering,” Woolley recalls. “He saw immediately the impact of this—he knew how important it would be. He said, ‘It just jumped out at me.'”
After further experiments to verify their findings and clarify the role of the new enzyme, Simmons published the results in the Proceedings of the National Academy of Sciences in 1991, announcing the discovery of a new form of cyclooxygenase, which would come to be known as COX-2. It turned out that the original COX investigated by Vane is actually two separate enzymes: COX-2 is to blame for some forms of pain and inflammation while COX-1 is responsible for protecting the stomach lining. That’s why aspirin and similar drugs like ibuprofen and naproxen—which inhibit both forms of COX—deaden pain and slow inflammation but also irritate the GI tract. Simmons’ publication, which was followed later that year by similar work by UCLA researchers, kicked off the race to develop new drugs that would slow down COX-2’s ability to produce molecules that cause pain and inflammation, while leaving COX-1 free to perform its helpful duties.
On Beyond COX-2
When Meredith L. Simmons, ’06, now a BYU pre-nursing student and a sophomore goalie on the soccer team, was growing up, her friends thought her dad was a nerd because he was a scientist. Now they ask her why he’s on TV news so often. She has had a lot of practice giving the answer: “Have you ever heard of Celebrex or Vioxx?” she asks them. “Well, those are COX-2 inhibitors, and my dad discovered COX-2.”
“I remember feeling like the new drugs were softer,” Trudy Simmons says. “They seem to alleviate the pain but don’t feel as harsh as the others I had been taking. I take some form of a COX-2 inhibitor every day now.”
In addition to a direct effect on human health, Simmons’ discovery also opened up a new horizon in biochemical research. Typing COX-2 into the search function on a medical research database yields more than 4,000 publications that have grown out of the first BYU study.
“COX-2 has changed that field,” says Xie, now a group leader at Celgene, a biotechnology company. “I do not think anything that I have done or will do can be compared with that in terms of significance. It is the opportunity of a lifetime to have your name associated with something like that.”
Research is underway exploring possible roles of COX-2 in cancer, Alzheimer’s disease, atherosclerosis, reproductive ailments, and neurological disorders like Lou Gehrig’s disease. Simmons has since focused his research on COX-2’s role in cancer and pain and was invited to spend the 1995–96 academic year as a visiting researcher at the William Harvey Research Institute, founded by Sir John Vane, who was knighted for his breakthrough research and who worked side-by-side with Simmons to further their collective discoveries.
Last fall Simmons announced in the Proceedings of the National Academy of Sciences a third form of COX, which appears to be the target for acetaminophen, the drug behind Tylenol and other aspirin-free pain relievers. Just as with aspirin three decades ago, physicians still don’t know how acetaminophen relieves pain, but Simmons is on the case. In March, BYU announced an agreement with Merck to license COX-3 to the pharmaceutical giant in return for future royalties from any products or services that arise from the research. Merck also agreed to fund future research in Simmons’ laboratory, now a large room full of gleaming instruments in the Ezra Taft Benson Building.
As demanding and detail-oriented as he is in the laboratory, Simmons is often easily distracted at home. Amber Simmons, 14, relates through laughter the time a caller asked for her father and she yelled for him to get the phone. “He walked into the kitchen and said, ‘Hello? Hello?’ and just stood there,” she says. “I waited and then I said, ‘Dad, you have to pick up the phone!'”
Meredith remembers occasions when her dad, lost in thought over a new obstacle in the lab, missed turns on the way to drop her off at school. Sometimes she has to say, “Dad. Dad. Dad!” to get his attention. “But he’s a great dad,” she says. “He always gives the best advice on any topic.”
On neutral territory, Simmons demonstrates a calm demeanor and a disarming curiosity about others, often manifest in one of his favorite phrases: “Let me ask you a question.” But in the throes of research or when discussing his circumstances vis-à-vis his competitors’, his eyes tend to widen, gestures get more dramatic, and tufts of hair find themselves sticking up on the top of his head.
“There’s a lot about Dr. Simmons that’s classic scientist,” says Jeffrey G. Chipman, ’91, who, as an undergraduate, worked with Simmons on the middle to latter stages of his COX-2 publication. Chipman credits Simmons’ demanding mentorship with helping him achieve his current position as assistant professor of surgery at the University of Minnesota, where he studies the role of COX-2 in trauma and critical care medicine. “He is very intense about his work.”

Although Simmons is pleased with his COX discoveries, he is not contentto rest on his laurels. His goal remains to make “clear contributions in a wide variety of areas.” Photo by Bradley H. Slade, ’94.
Chipman was on the receiving end of that intensity during his experience in Simmons’ lab. The professor, anxious to finalize and publish his discovery of the new enzyme, pulled his undergraduate assistant—his only help other than Xie—into his office and explained that the student’s current level of performance was inadequate. “That was a defining point in our relationship—it motivated me and I started to pay more attention in the lab. Even though he was paying me, he didn’t want it to be a job; he wanted it to be an academic experience. I ended up functioning like a graduate student when I was a junior and senior in college.” Chipman’s undergraduate honors thesis was named the best at the university, and he was giving lectures to physicians on the new COX-2 inhibitors when he was still a medical resident.
Simmons tries to temper his innate drive with concern for individual students. He typically trains four to six undergraduates at a time. Each one pursues his or her own research project that is expected to result in a scientific publication. A strong recommendation from Simmons is often a passport to acceptance in a top-flight medical school or other graduate program.
“Dr. Simmons had a palpable and contagious excitement for research. He drew momentum from asking the kind of questions that had the potential of unearthing the big find,” says Bryan A. Ballif, ’93, one of Simmons’ former students who, after completing a PhD at Harvard under one of Simmons’ former colleagues there, is doing post-doctoral work at Seattle’s Fred Hutchinson Cancer Research Center. “My working relationship with him was unique, being at the same time formal and collegial. I both respected him as a distinguished mentor and yet felt like one of his peers.”
That Simmons can achieve what he does while relying so heavily on undergraduates and a few graduate students and post-doctoral fellows amazes Woolley, the dean of Simmons’ college.
“He’s competing against labs of a hundred people. Why should Dan be able to do this?” Woolley wonders. “You would not expect this to come out of a place that doesn’t have a medical school.
“Other labs have 30 post-docs and 30 graduate students, a medical support team, and technicians. Dan was the brains and the brawn behind this, with a few students. It’s Dan’s drive that makes it work, that has made the students productive, and that has made his colleagues more productive. It’s Dan’s vision and tenacity—that’s really what it is—and the inspiration that goes with it.”
Seeking Competent Science
At the beginning of his master’s program at BYU, Simmons was asked by an instructor to set a goal for his career. The response he wrote in his notebook was “to do science that others judge to be competent.” That objective has guided his life’s work.
“Science is a really confusing thing. You never know whether you’re doing well or you’re going to the dogs. This morning, I was about ready to jump off the Benson Building,” says Simmons, apparently fond of jokes about departing his laboratory in unorthodox ways. “I got the crude raw negative data on a really critical experiment, and I’m going nuts. I just want to chuck it all. In the afternoon, another experiment comes in that looks a little more encouraging, so I was willing to stay on the top floor. That’s exactly how it is. You don’t know really if you’re doing well or if you’re failing most of the time. Although occasionally you have those experiences where you look at that COX>-2 and you know you’ve just hit a gold mine.”
Despite that “gold mine”—Simmons and BYU have yet to reap financial rewards from his discoveries—Simmons still believes he has not achieved his goal.
“I need greater productivity,” he says. “I’ll have done it when I have made a number of very clear contributions in a wide variety of areas. That may be a goal I never achieve, but I think it’s a worthy one for all scientists.”
Although Simmons may not be satisfied with his efforts to meet his own stated standards, someone else is. With the brevity that comes as a license to reclusive Nobel laureates, Vane says simply of Simmons, “He’s done very well.”
Michael Smart is a media relations manager for BYU University Communications. His article “Jack Sites’ Creepy Friends,” in the winter 2003 issue of BYU Magazine, received a silver medal for “Best Articles of the Year” in a recent national writing competition.
Feedback: Send comments on this article to magazine@byu.edu.









